The Science Behind Mice Against Ticks
Technology Overview
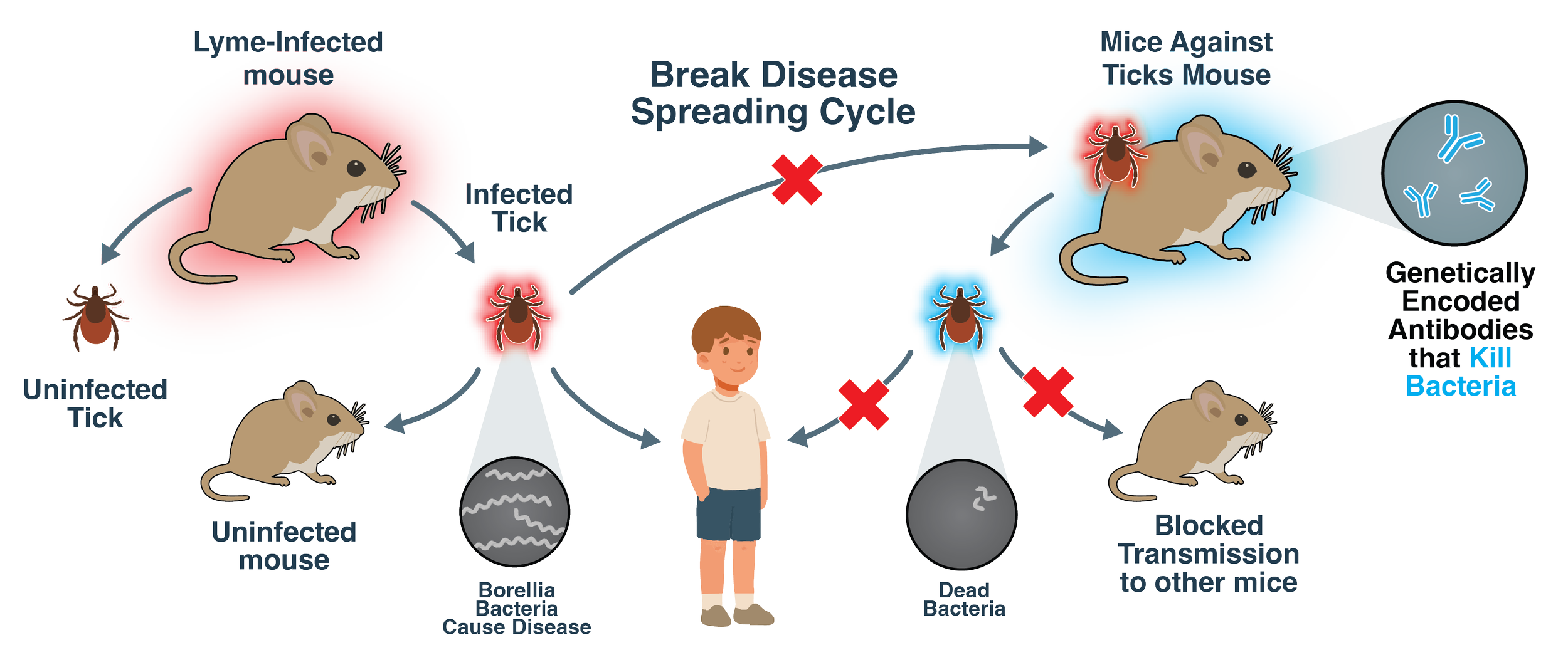
Engineered mice produce protective antibodies that stop Lyme-causing bacteria from infecting ticks, helping break the cycle of transmission to people and wildlife.
Major Accomplishments
With support from the Mice Against Ticks nonprofit and funding from philanthropic donors, the research team has exceeded key scientific and technical goals by:
Developing the first genetically engineered white-footed mice (Peromyscus leucopus) — the primary reservoir of Lyme disease — using novel, low-cost estrous tracking technology and embryo manipulation protocols.
Demonstrating proof-of-concept for heritable Lyme immunity by engineering Mus musculus to express a protective anti-Borrelia antibody that prevents Lyme infection and blocks tick transmission.
Isolating the first potent anti-Lyme antibodies from white-footed mice in partnership with Dana-Farber Cancer Institute, creating a species-specific therapeutic foundation for future engineered lines.
Producing two patent applications and a landmark publication demonstrating engineered heritable immunity as a viable strategy for interrupting the Lyme disease cycle, with the potential to reshape reservoir-borne disease control.
Building a cross-institutional collaboration among MIT, Dana-Farber, Tufts, and Duke to unite mouse engineering, antibody discovery, ecological planning, and responsible biotechnology development.
Current work
With foundational tools now established, the team is actively working to advance Lyme disease prevention while laying the groundwork for solutions to other reservoir-borne diseases. Here are the team’s near-term goals:
Generate the first Lyme-immune white-footed mice.
Perform functional testing of engineered mice in containment settings to evaluate protection, safety, and inheritance across generations.
Design and launch ecological safety studies in collaboration with local scientists and residents.
Advance regulatory, biosafety, and community engagement frameworks to support potential future release scenarios, guided by transparency and local governance.
Refine antibody engineering strategies and explore additional reservoir-targeted approaches to expand protection against tick-borne diseases.
How to Support Our Research
Federal grants have historically overlooked Lyme disease. Your donation helps fill that gap and drives the science forward.
How Do You Test Lyme Immunity?
Our mice are tested through tick challenges, which recreate the natural transmission cycle of Lyme disease in the lab. It’s a complex, months-long process that involves three players: mice, ticks, and the Lyme bacterium.
First, infected ticks are placed on the mouse to see if it becomes infected. Later, “clean” ticks that are free of infection are allowed to feed on the same mouse. If those clean ticks don’t pick up Lyme bacteria, we know the mouse’s immune system successfully stopped transmission, meaning we’ve created a Lyme-immune mouse!
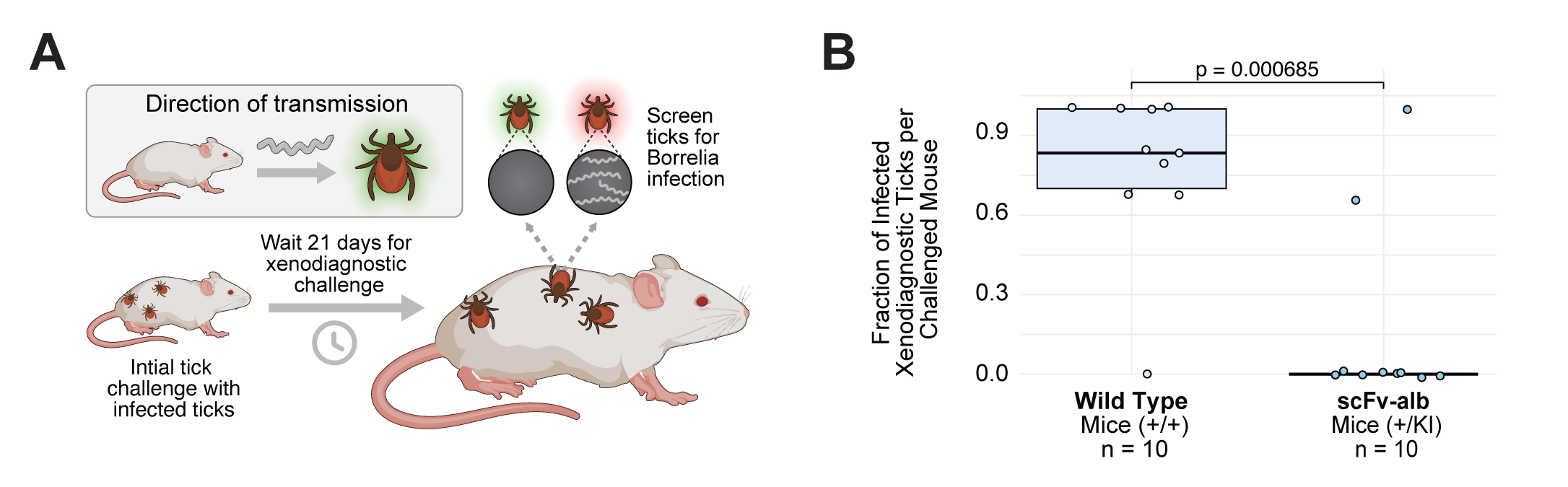
Xenodiagnostic Challenge and Infection Analysis in Antibody Expressing Mice
A) Schematic of the xenodiagnostic challenge. Uninfected larval ticks were fed on previously challenged antibody expressing mice 21 days post-challenge. B) Fraction of infected ticks per mouse, assessed by indirect immunofluorescence of dissected tick guts post-molt. Detection of Borrelia burgdorferi spirochetes in any tick was considered evidence of an infected and infectious mouse.
Publications
Our work is grounded in science — and made possible through collaboration with leading academic researchers. The Mice Against Ticks project began as a scientific inquiry and continues to advance through rigorous, peer-reviewed research.
This page will serve as a growing archive of the publications, data, and discoveries generated by our collaborators.
Buchthal, J. et al., 2024. Heritable Immunization Establishes a New Model for Pathogen Control. Bioengineering. Available at: https://www.biorxiv.org/content/10.1101/2024.12.19.629026v1
Buchthal, J. et al., 2023. Low-cost camera-based estrous tracking enables transgenesis in Peromyscus leucopus, the primary reservoir for Lyme disease. bioRxiv, p.2023.10.20.563285. Available at: https://www.biorxiv.org/content/biorxiv/early/2023/10/22/2023.10.20.563285
Buchthal, J. et al., 2019. Mice Against Ticks: an experimental community-guided effort to prevent tick-borne disease by altering the shared environment. Philosophical transactions of the Royal Society of London. Series B, Biological sciences, 374(1772), p.20180105. Available at: https://royalsocietypublishing.org/doi/10.1098/rstb.2018.0105
Goethert, H.K. et al., 2021. Retrotransposon-based blood meal analysis of nymphal deer ticks demonstrates spatiotemporal diversity of Borrelia burgdorferi and Babesia microti reservoirs. Applied and environmental microbiology, 87(2). Available at: https://doi.org/10.1128/AEM.02370-20
Noble, C. et al., 2019. Daisy-chain gene drives for the alteration of local populations. Proceedings of the National Academy of Sciences of the United States of America, 116(17), pp.8275–8282. Available at: https://www.pnas.org/doi/10.1073/pnas.1716358116
Telford, S.R., 3rd, Buchthal, J. & Elias, P., 2019. Early questing by Lone Star tick larvae, New York and Massachusetts, USA, 2018. Emerging infectious diseases, 25(8), pp.1592–1593. Available at: https://pmc.ncbi.nlm.nih.gov/articles/PMC6649318/